BMP Medical Kicks Off DelmiaWorks Implementation
In 2021, BMP Medical began the process of implementing an DelmiaWorks manufacturing ERP execution MES/data-collection software. By mid-2022, the implementation is expected to be completed. DelmiaWorks real-time monitoring, project management, and cloud-based key features will allow BMP Medical to better serve leading medical device OEMs. For Real Time Management, Advanced Manufacturing, Financial Management, MFG Manufacturing & Scheduling, CRM & Sales Order Management, Quality & Compliance Management, Distribution & Warehouse Management, and every operation in between, DelmiaWorks will assist in the automation of our facility and shop floor processes. DelmiaWorks is considered the gold standard and has been exclusively focused on the manufacturing industry for almost 25 years.
Employees have been engaged in the training phase of the process in our new, on-site training facility. DelmiaWorks will eliminate manual processes and redundancies, reduce inventory carrying costs, increase operational efficiencies, protect brand integrity, and produce concise and accurate accounting.
“We are excited to expand our commitment to ensuring customers’ success with the launch of the DelmiaWorks program,” said Michael Faulkner, Chief Executive Officer & President of BMP Medical. “We are eager to optimize the DelmiaWorks software so we can maximize growth and profitability for our customers. The insights and efficiencies gained from DelmiaWorks will help us remain a global leader in customer plastic manufacturing.”
“The DelmiaWorks process is helping us to further streamline our program management systems and company-wide processes, and also to strengthen customer relationships and create new customer opportunities,” said John Faulkner, Sales & Program Manager.
About BMP Medical
BMP Medical provides plastic contract manufacturing services to leading medical device and diagnostic customers in need of injection molding and injection-blow molding. BMP Medical’s 80,000 sq. ft. facility is headquartered in Sterling, Massachusetts. BMP Medical is a global contract manufacturer servicing the Medical Device and Medical Diagnostic markets in all areas, including, but not limited to, IVD, IV access, sample-preparation devices, and lateral-flow technologies. BMP Medical can service our partner requirements on a worldwide basis. Certifications: ISO13485:2016; FDA registration #1220502; ISO Class 8 injection molding; ISO Class 8 assembly & packaging; 21 CFR 820 Quality System Regulation; FDA registered; and MedAccred certified. Services Offered: Class 7 Assembly, Clean Room Manufacturing (Class 8), Clean Room Packaging and Assembly (Class 8), Custom Assembly & Packaging, Custom Injection Blow Molding (IBM), Custom Injection Molding, DNase- and RNase-free manufacturing, FDA Registered Facility, Product design & development services, R&D tooling, and Sterilization services.
This plastic injection molding process guide is intended to provide useful information about injection molding considerations, processes, the primary work leading up to getting a mold on a press, and the many caveats that go into producing a finished molded part. We hope you find this guide useful. It is intended for potential customers, engineers, designers, and outsourcing purchasing professionals.
What is plastic injection molding?
Simply stated, the plastic injection molding process injects heated plastic substrates into a metal-tooled cavity of the desired shape and it is allowed to dry, cure, and set.
But not so quick—let’s do a deeper dive into the step-by-step processes and the caveats evolved throughout the entire process to manufacture a finished plastic injection-molded medical device.
The plastic injection molding manufacturing process is ideal for consistently producing finished components or parts in a cost-effective manner. The up-front tooling process can be rather costly, so it is really only suitable for medium- to large-volume manufactured parts. This manufacturing process produces very little material waste, and the leftover thermoplastics can be recycled.
SIM
Scientific injection molding (SIM) is a data-driven, scientific approach. Cycle times are minimized, machine efficiencies are improved, and productivity is increased. Scientific injection molding is a strong tool supporting the objectives and practices of a lean manufacturing culture. SIM reduces or eliminates many of the forms of waste by providing a robust, predictable molding process.
Plastic injection molding guide process:
First, based on your CAD drawings and 3D printed prototype, a decision needs to be made on which injection molding process is best.
Commonly used plastic manufacturing techniques
The most commonly used plastic manufacturing techniques are injection molding, injection blow molding, two-shot molding, insert molding, rotational molding, compression molding, extrusion molding, and thermoforming. The requirements and specifications of your finished product will determine which injection molding process is best for your finished part.
What is plastic injection molding?
Injection molding is ideal for many small precision medical and large automotive part applications. This is the most commonly used plastic manufacturing process, and it offers a great deal of flexibility in material choices that can change the appearance of the final product.
What is plastic injection blow molding?
Injection blow molding is used in manufacturing parts that need to be hollow and maintain a uniform wall thickness. This process utilizes the blowing of air into the thermoplastic cavities until the desired shape is formed. Bottles are a common product manufactured by this method.
What is plastic two-shot molding?
Two-shot molding has a few names, it is also known as 2-shot, dual-shot, and double-shot molding. This process combines two parts into a single molded part by combining two machining processes. It offers flexibility for combining different resins materials together.
What is plastic insert molding?
This is a single-step process where two parts are formed together. This process is best suited for parts that need to form together tightly.
Plastic injection molding guide considerations:
Key considerations for the manufacturing process selection and thermoplastics material choice:
- Overall design
- Functionality
- Shelf life of the product
- Cost
- Tensile strength
- Shock absorbance
- Impact tolerance
- Scratch resistance
- Heat resistance
- Performance
- Stability
- Chemical resistance
- Final product weight
- UV protection
Plastic injection molding guide Thermoplastic Material Selection
Which medical-grade thermoplastic should be used for manufacturing the medical product?
- Polyethylene
- Polypropylene
- Polystyrene
- Polymethyl methacrylate
- Polyvinyl chloride
- Polyamides (nylons)
- Acrylonitrile butadiene styrene (ABS)
- Polycarbonate
Our blog Common Thermoplastics in Injection Molding (https://www.bmpmedical.com/blog/common-thermoplastics-in-injection-molding/) provides an in-depth review of the different thermoplastics used in medical products, their key benefits, and their tensile strength in psi. Given the precision required in most medical devices, it is generally best to avoid resins that have asymmetrical shrinkage.
Injection Molding Tooling
Mold tooling is essential in producing an accurate and cost-effective manufactured product.
These key tool-making considerations need to be made before the injection molding process can begin:
- Cooling compensation
- Tool mold size and material selection
- Designing, building, and debugging the mold
- Gate placement
- Polishing process, mold fitting, and assembly
- Mold fitting and trial prototyping
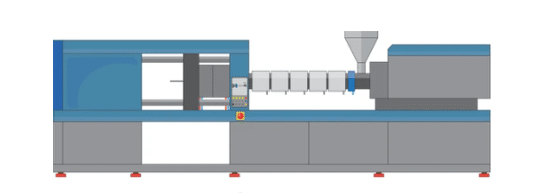
How the injection molding press works
The Injection molding manufacturing process
Injection molding is a very complex process. The actual machine has three major parts: the injection unit, the mold section, and the clamp. Thermoplastic pellets are fed into the hopper of the unit and then pushed into the barrel. A turning screw pushes the thermoplastic pellets forward. Heater bands surround the injection unit, melting the thermoplastic pellets. The screw continues to turn, pushing the molten into the open cavity area of the mold. It opens and the part is ejected. The mold closes and the process repeats.
Injection molding monitoring is an ongoing process
The machine operator and engineer continuously monitor the following predetermined parameters to deliver a quality product:
- Injection plasticizing pressure
- Mold, nozzle, air, melt, and cooling temperature
- Filling time
- Packaging and holding time
Why BMP Medical should be your next plastics manufacturing partner
Located in the United States, BMP Medical is near Leominster, Massachusetts, where Pioneer Plastic City was founded, and the region has many thriving plastic manufacturing companies.
BMP Medical provides plastic contract manufacturing services to leading medical device and diagnostic OEMs in need of injection molding, injection blow, two-shot, and insert molding. BMP Medical’s 80,000 sq. ft. facility is located in Sterling, Massachusetts. We foster strong trust-driven relationships to develop inventive solutions tailored to customer and end-user needs.
From concept through commercialization, BMP offers in-depth problem-solving backed by deep industry knowledge and operational excellence.
BMP Medical is a global contract manufacturer servicing the Medical Device and Medical Diagnostic markets in all areas, including, but not limited to, IVD, IV access, sample-preparation devices, and lateral-flow technologies. Headquartered in Sterling, Massachusetts, BMP Medical can service our partner requirements on a worldwide basis.
Certifications: ISO13485:2016; FDA registration #1220502; ISO Class 8 injection molding; ISO Class 8 assembly & packaging; 21 CFR 820 Quality System Regulation; FDA registered; MedAccred Certified
Services Offered:
- Class 7 Assembly
- Clean Room Manufacturing (Class 8)
- Clean Room Packaging and Assembly (Class8)
- Custom Two-shot Molding
- Custom Assembly & Packaging
- Custom Injection Blow Molding (IBM)
- Custom Injection Molding
- Custom Insert Molding
- DNase- and RNase-free manufacturing
- FDA Registered Facility
- Pad printing
- Product design & development services
- R&D tooling
- Sterilization services
Have a question for our technical team on the plastic injection molding process?
Get to know BMP Medical today.
Over the past several years, the United States has seen a growing demand for reshoring medical device and component manufacturing. The past year has done much to prove that reshoring manufacturing is even more critical than we ever thought. With businesses quickly pivoting and supply chains being pushed like never before, there is a greater need for companies to better control many aspects of their medical devices and components, such as design, development, manufacturing, and assembly.
Key Factors to choosing a medical device manufacturing partner
When choosing a U.S.-based medical device and component manufacturing partner, several key factors determine the right fit for your product and business, such as quality control/quality assurance (QMS), the complexity of the product, IP protection, better supply chain management, lower shipping costs, mitigation of risk, avoidance of unanticipated import challenges and taxes, better communication, lower travel and communication costs, greater market appeal, and more jobs for the U.S. economy.
Things to consider with selecting a medical device contract manufacturer
Medical device manufacturing • Quality control/quality assurance (QMS)
Quality control and quality assurance are paramount in medical device design, development, and manufacturing. An important first step is being able to visit the contract manufacturing company that you’re considering partnering with. Virtual tours are another good option. It is crucial to understand the detailed quality control and quality assurance tactics that will be put into place to deliver a flawless, well-documented finished product throughout each stage of the design, development, and manufacturing process.
Both quality control and quality assurance fall under QMS. Making sure your medical device manufacturing partner is embracing real-time data QMS procedures will help with evolving compliance and regulations requirements. Finding out what robust QMS they have in place is important, and it is imperative to mitigate risk at every stage of your product lifestyle. Several certifications and accredited programs, including MedAccred, FDA-compliant, ISO-certified, and adherence to cGMP practices, should be taken under consideration to mitigate risk.
Complexity of product
It is vital to find a design, development, and manufacturing medical device contract manufacturing partner that has the proper equipment, technology, and expertise, and is capable of meeting your production levels throughout the entire product life cycle while maintaining outstanding production of high-quality throughput. Very important also is asking the right questions and developing a cohesive understanding as to what resources would be readily available to deliver a high-quality medical device or component on time, on budget, and on schedule.
Medical device manufacturing • IP Protection for your innovations
Protecting your medical device is an important action that should be considered at the inception of the development cycle. The medical device industry is a crowded space with a lot of pending patents and registered designs. You must engage with a patent attorney early on and obtain a signed NDA with any manufacturing company you engage in discussions with. Many OEMs have expressed some concerns around IP protection when working with an international medical device manufacturer.
Medical device manufacturing • Better supply chain management
Consider the resources needed to manage a global supply chain from a sourcing and logistics standpoint when selecting a medical device manufacturing partnership. These additional internal resources should be calculated into your overall project cost. How will a global supply chain affect your lead time? Could unexpected shipping delays affect your product deliverable dates?
Unanticipated import challenges and taxes
China has seen seeing rising labor rates over the past several years and has also experienced importing challenges that delay delivery times. China is also hampered by additional shipping costs, uncertainty around tariffs, and rising taxes. Many U.S.-based manufacturers have been able to bridge the cost-efficiency gap with investments in automation, technology, and enhanced operational programs, all with a keen eye on delivering a higher-quality product.
Mitigating risk
Greater regulatory requirements have put more importance on mitigating risk in order to deliver safer and more effective medical devices. Companies have put greater emphasis on developing a risk management plan that analyzes, evaluates, controls, and monitors risks. Does your potential medical device manufacturer have a robust QMS? Do they hold the needed certifications? How will audits be performed? Does your potential manufacturing vendor have a crisis response plan that ensures manufacturing continuity?
Better communication, less travel, and lower communication costs
Working with a medical device manufacturing vendor in the same hemisphere that can respond to production changes in a time-sensitive and responsive manner is an added advantage. Even in our digital age, human interaction is a vital component to delivering great customer service.
Over the past seven years, more and more OEMs are returning their medical device design, development, manufacturing, and assembly work to the United States, which provides greater market appeal and adds jobs to the U.S. economy.
Why BMP Medical should be your next plastics manufacturing partner
Located within the United States, BMP Medical provides plastic contract manufacturing services to leading medical device and diagnostic OEMs in need of injection molding, injection blow, two-shot, and insert molding. BMP Medical’s 80,000 sq. ft. facility is located in Sterling, Massachusetts. We foster strong trust-driven relationships to develop inventive solutions tailored to customers and end-user needs. From concept through commercialization, BMP offers in-depth problem-solving backed by deep industry knowledge and operational excellence. BMP Medical is a global contract manufacturer servicing the Medical Device and Medical Diagnostic markets in all areas, including, but not limited to, IVD, IV access, sample-preparation devices, and lateral-flow technologies. Headquartered in Sterling, Massachusetts, BMP Medical can service our partner requirements on a worldwide basis.

Certifications: ISO13485:2016; FDA registration #1220502; ISO Class 8 injection molding; ISO Class 8 assembly & packaging; 21 CFR 820 Quality System Regulation; FDA registered; MedAccred Certified
Services Offered:
- Class 7 Assembly
- Clean Room Manufacturing (Class 8)
- Clean Room Packaging and Assembly (Class8)
- Custom Two-Shot Molding
- Custom Assembly & Packaging
- Custom Injection Blow Molding (IBM)
- Custom Injection Molding
- Custom Insert Molding
- DNase- and RNase-free manufacturing
- FDA Registered Facility
- Pad Printing
- Product Design & Development Services
- R&D Tooling
- Sterilization Services
It is time to bring design, development and manufacturing back to the United States. Get to know BMP Medical today.
Understanding what are the most common thermoplastics used in medical device injection molding is very important. Selecting the correct medical-grade plastic material for a project is a critical decision in manufacturing the perfect part. When working with an injection molding CMO medical device and component partner, it is important to work with injection molders that adhere to stringent manufacturing standards.
It has long been considered that polymers have a significant advantage over metals in the context of medical applications because the isotonic saline solution that makes up the body’s extracellular fluid is extremely hostile to metals, but it is not normally associated with the degradation of many synthetic high-molecular-weight polymers.
Polymers are typically classified into three groups
Thermoplastics
Thermoplastics are linear or branched polymers that can be melted with heat. They can be molded and removed using conventional techniques. Reheated wax can be molded into a different shape.
Thermosets
Thermosets are cross-linked polymers that are normally rigid and intractable. They consist of a three-dimensional molecular network. Thermosets cannot be remelted. They degrade rather than melt upon heating.
Elastomers (rubbers)
Rubbers are materials that exhibit elastomeric properties. They can be stretched to extension and will spring back when the stress is released.
Thermoplastics represent 90% by weight of all plastic used worldwide. Unlike most thermoset plastics, thermoplastics are processable without any serious loss of properties.
Common thermoplastics in injection molding
Common polymers and their medical uses
Polyethylene - - common thermoplastics used in injection molding for medical devices
This is by far the most popular plastic used worldwide. It is also called polythene. Polypropylene is 34% of all plastic used. It can be used for low- and high-density products. Generally speaking, high-density polyethylene (HDPE) is much more crystalline, has a much higher density, and is often used in completely different circumstances than low-density polyethylene (LDPE). Polyethylene is considered thermoplastic, but with modifications, it can behave as a thermoset. It is a cost-effective medical-grade material. Polyethylene absorbs almost no water, and it cannot be imprinted or bonded with adhesives without pretreatment. Like other synthetic plastics, it is not readily biodegradable. But it is an ideal material for sensitive medical equipment, devices, and supplies used every day in hospitals. This ideal medical-grade plastic is corrosion-resistant and retains its overall performance and structural integrity after frequent sterilization cycles.
Its high impact resistance, resistance to chemicals, and low moisture absorption make it a choice for medical-grade devices and components. Polyethylene doesn’t fade or retain dangerous bacteria, and it can withstand harsh cleaning agents. It is often one of the materials used in medical implants because it is a porous synthetic polymer that is biologically inert and does not degrade in the body.
Polyethylene is a very versatile material and is often referred to as the “steel” of plastics because it can so easily be customized for a variety of different uses. It is commonly used for containers, bottles, and many tubing applications, but it is vulnerable to UV radiation and is flammable. It has a tensile strength of 4,000 psi.
Product benefits of polyethylene include
- chemical resistance
- eco-friendly
- easy to clean
- flexible
- good stability
- heat resistance
- impact resistance
Polyethylene is commonly used for tubing applications, connectors, bottles, and plastic surgery implants.
Polypropylene - - common thermoplastics used in injection molding for medical devices
Polypropylene is a thermoplastic polymer used in a wide variety of applications. It is a white, mechanically rugged material and has a high chemical resistance. Polypropylene is used for protective packaging and medical equipment is tough and durable and can be recycled down from cars and households. It can be melted and reformed into plastic pellets that then are used to make new products. In fact, polypropylene bottles and containers are collected for recycling in most curbside programs across the country. Recycling polypropylene helps keep this superhero out of landfills.
Polypropylene’s characteristics make it ideal for tough, robust products, ranging from protective car bumpers to lifesaving medical tools to cold-weather gear for our soldiers. It has a very high tensile strength of 4,800 psi.
Product benefits of polyamide include
- stress resistance
- crack resistance
- great fatigue resistance
- good chemical resistance
- high performance
- good impact resistance
- high melting point
Within these different thermoplastic categories, there are thousands of variations to choose from. Ultimately, every OEM needs to consider the material’s hardness, flexibility, weight, and overall cost. Many factors come into play when selecting a thermoplastics to be used for medical device injection molding.
Polypropylene is commonly used to manufacture disposable syringes, membranes for membrane oxygenators, connectors, finger-joint prostheses, nonabsorbable sutures, reusable plastic containers, pharmacy prescription bottles, and clear bags.
Polystyrene
Polystyrene is one of the most widely used plastics. It is an inexpensive resin per unit weight. It is a rather poor barrier to oxygen and water vapor, and it has a relatively low melting point. Polystyrene is generally a glassy transparent and hard type of polymer. It is produced by the radical polymerization of styrene and can be solid or foamed. Polystyrene Petri dishes and other laboratory containers such as test tubes and microplates play an important role in biomedical research and science. For these uses, articles are almost always made by injection molding, and often sterilized after molding, either by irradiation or by treatment with ethylene oxide. It has a very high tensile strength of 3,600 psi.
Polystyrene is used for a range of medical applications, including test tubes, Petri dishes, diagnostic components tissue-culture trays, protective packaging, and disposable plastic cutlery.
Polymethyl methacrylate
Polymethyl methacrylate (PMMA) is a synthetic resin produced from the polymerization of methyl methacrylate. A transparent and rigid plastic, PMMA is often used as a substitute for glass. PMMA is a tough and rigid plastic. In addition, it has almost perfect transmission of visible light. And because it retains these properties over years of exposure to UV radiation and weather, it is an ideal substitute for glass. Because PMMA displays the unusual property of keeping a beam of light reflected within its surfaces, it is frequently made into optical fibers for telecommunication and endoscopy.
It is commonly used to make bone cement, artificial teeth, implanted teeth, denture materials, dental fillings, intraocular lens, and membrane for dialysis or ultrafiltration.
Polyvinyl chloride - - common thermoplastics used in injection molding for medical devices
Polyvinyl chloride (PVC) is one of the most commonly used thermoplastic polymers in the world. It is used most commonly in the construction industry, but is also used for signs, healthcare applications, and as a fiber for clothing. PVC is produced in two general forms: (1) rigid or unplasticized polymer (RPVC) and (2) flexible plastic. Flexible PVC is commonly used in construction as insulation on electrical wires or in flooring for homes, hospitals, schools, and other areas where a sterile environment is a priority, and in some cases as a replacement for rubber. Rigid PVC is also used in construction as pipes for plumbing and for siding.
Some of the most significant properties of polyvinyl chloride (PVC):
Density: PVC is very dense compared to most plastics (specific gravity around 1.4)
Economics: PVC is readily available and cheap.
Hardness: Rigid PVC is very hard.
Strength: Rigid PVC has extremely good tensile strength.
PVC is commonly used to manufacture disposable medical articles, hemodialysis or hemoperfusion, blood tubing line, cardiac catheters, blood bags, and artificial limb materials.
Polyamides (nylons) - common thermoplastics used in injection molding for medical devices
Polyamide, also known as nylon, is a synthetic thermoplastic polymer. It offers an extremely broad range of available properties and is used in cars, combs, pump parts, screws, and in films for food-packaging needs. It is often used as a substitute for low-strength metals, and because of its strength, inflexible nature, temperature resilience, and chemical compatibility.
Polyamide plastic is good for CNC machining, injection molding, and 3D printing. It has a higher impact-strength factor compared to polystyrene, or polycarbonate. And its strength factor can be increased even more by a process called “conditioning” and/or be combined with other materials to improve its overall strength. It is a good option for parts that see a lot of wear and tear.
The range of polyamides available offers many advantages. Nylons tend to provide good resistance to most chemicals. However, it is one of the trickiest materials to mold and requires a strong processing plan in place to prevent variations, defects, and excessive waste. Polyamide in most cases is considerably more costly. It has a very high tensile strength of 76 MPa (11,000 psi).
Product benefits of polyamide include
- excellent chemical resistance
- great wear resistance
- great fatigue resistance
- good chemical resistance
- high performance
- good impact resistance
- abrasion resistance
- good for short injection cycles
Nylon (polyamide) fibers are used in textiles, fishing lines, and carpets. Nylon films are used for food packaging, gears and bearings (because of its self-lubricating properties), nylon-bristled toothbrushes, electrical insulations, and cable ties.
Acrylonitrile butadiene styrene (ABS)?
Acrylonitrile butadiene styrene (ABS) is a very common thermoplastic polymer and is ideal for very vigorous critical applications. It is commonly used in part production and 3D-print manufacturing for OEM manufacturers. Essentially, the three https://www.merriam-webster.com/dictionary/constituents materials provide a balance of properties: butadiene units impart good impact strength; acrylonitrile units afford heat resistance; and styrene units give the co-polymer its rigidity. ABS is regarded as a good engineering plastic (that is, a substitute for metals in structural parts). It can be injection molded, blow molded, or extruded. And it is also gamma & EtO sterilizable.
The chemical makeup of ABS makes it easy to melt down and reshape repeatedly without degrading its chemical structure. It is a recyclable plastic, and it is one of the easiest plastics to handle because it cools down and hardens quickly.
It is ideal for manipulating with additional machining, painting, gluing, sanding, and so forth. ABS is not very chemical-resistant and shouldn’t be considered for applications that require UV resistances or electrical insulation. ABS has a tensile strength of 5,500 psi. Common thermoplastics in injection molding for medical device
Product benefits of ABS include
- good tensile strength
- low cost
- shock absorbance
- strong impact
- scratch resistance
- heat resistance
Common uses of ABS are nonabsorbable sutures, tendon prostheses, drug-delivery systems, tracheal tubes, safety helmets, cleaning applications, and housings.
Polycarbonate - common thermoplastics used in injection molding for medical devices
Polycarbonate is a group of thermoplastic polymers that contain carbonate groups within the overall chemical structure. It is naturally transparent to visible light and offers good UV protection. It is often used for eyewear lenses and is considered to be a good alternative to glass. Polycarbonate is a very strong material and is relatively shatterproof and is also a common material used in medical devices.
Many polycarbonate grades are used in medical applications. Medical grades can be sterilized using steam at 120° C, gamma radiation, or by the ethylene oxide (Eto) method.
Polycarbonate is four times stronger than fiberglass and gets its strength from its flexibility, so it gives a little upon impact. It ranges anywhere between 35% and 300% more expensive than acrylic. Its strength is measured in MPa instead of psi. It has a very high tensile strength of 70 MPa (10,152.6 psi).
Product benefits of polycarbonate include
- chemical resistance
- electrical resistance
- good stability
- good chemical resistance
- high performance
- high heat resistance
- impact resistance
- lightweight
- good UV protection
Common uses of polycarbonate are protecting the eye from UV light, lighting lenses, sunglass/eyeglass lenses, swimming goggles, and scuba masks.
Do you have a question for our team about common thermoplastics used in injection molding for medical devices? Contact us today.
BMP Medical is your one-stop for custom plastic injection
and injection blow molding for medical devices.
For more than 40 years, we have partnered with leading medical device OEMs to create custom plastic injection molding solutions in both finished medical devices and components. During our discovery process, we review and discuss certain criteria that will lead to the success of your finished injection molded part or packaged finished medical device.
Our BMP team and OEMs collaborate to develop a mutual understanding of several factors that drive the material selection and the actual injection molding process for each manufactured medical device and/or component.
Some of those factors:
- Environment
- Chemical compatibility
- The complexity of finished part or device
- Mechanical function
- Physical load
- Sterilization
- Thermal conditions
BMP works with you closely, from prototypes through full-scale production. Working with an experienced full-time injection molding manufacturing partner will give you greater control over the design, from manufacturing through process development. You can also count on high-quality parts for finished medical devices for optimal performance—and you benefit from a lower total manufacturing cost. Getting BMP involved in the development cycle early is crucial in establishing the needed design requirements to help reduce lead time and overall costs.
Our BMP plastic injection molding capabilities include injection molding, injection blow, 2-shot (two-shot molding), and insert molding (overmolding).
- Injection molding
Injection molding can be the ideal option for highly engineered, small precision parts that are large-volume and require multicavity options. Several key factors discussed in the discovery process will help to determine if injection molding is the method of choice for your project.
- Blow molding
Blow molding can be used to create numerous types of stand-alone hollow parts. A single-constructed hollow piece with shapes can be created uniformly and economically. The overall wall thickness variation is often a critical and deciding factor in using this process or not.
- 2-shot molding (two-shot molding)
2-shot molding is a designated process that allows the medical device or component to be molded and overmolded, all within a single cycle. In the first step, the initial shape is molded, and then the remaining open space is injected to provide a different texture and/or color. With higher-volume programs, this process can offer some cost savings.
- Insert molding (overmolding)
Insert molding is a great molding option: it can eliminate the need for assembly in some cases, allows for areas of color contrast within the given medical device and or component, and can provide greater design aesthetic to the finished medical device or component.
Do you have a question for our plastic injection molding team? Contact us today.
Medical plastic products have become more advanced as the global market for high-quality medical devices continues to expand. Such devices have become a necessary and vital component in the modern healthcare system. Medical plastic products are available in a wide assortment of devices. From test equipment like vials and beakers to surgical instruments, catheters, and implants, plastics are used more and more for their high performance, lightweight, and lower costs.
Creating Safe and Effective Medical Devices
Diverse medical applications, product durability, and biocompatibility are all important factors that original equipment manufacturers (OEMs) of medical plastic products must consider to meet market demands. Just as important, medical-grade plastic materials must meet regulatory requirements throughout the globe. To ensure that the plastic material is safe and effective for medical products, polymers for medical devices are generally made of thermoplastic materials.
- Thermoplastic is a type of synthetic polymer. All plastics are made from specific types of synthetic polymers, and the terms ‘polymer’ and ‘plastic’ are generally used synonymously. That said, thermoplastics are distinguished from other synthetic polymers because they can be reheated and remolded time and again without irreversible degradation. The changes are physical, not chemical, which means thermoplastics can be reused and recycled. When heated, thermoplastics liquefy, and the material becomes easily moldable, then solidifies into a durable finished product when cooled. This makes thermoplastics ideal for custom plastic injection molding and injection blow molding services.
- The choice of thermoplastic materials must be of medical-grade polymers. The properties of medical-grade plastics or polymers share common characteristics, meaning they must be manufactured under a physician’s license to pass the verification and validation requirements of the regulatory agencies. For specific applications regarding patient safety, they must offer biocompatibility. Further, the finished product must be temperature, impact, and corrosion resistance to withstand the high wear and repeated sterilization cycles medical plastic products are subject to. Common thermoplastic materials used in the manufacturing of medical plastic products include polycarbonate, polypropylene, polyethylene, or the formulation of custom polymers to meet specific medical device applications.
- Polycarbonates are used in a variety of materials, but are most well-known for their impact resistance properties and withstanding high-temperature ranges. Applications for polycarbonate polymers include plastic lenses in eyewear, automotive components, protective gear, and medical devices. Because polycarbonates have good heat-resistant properties, they are very pliable and can be formed at room temperature without cracking or breaking. Such properties make polycarbonate very useful in prototyping applications, such as with medical devices.
- Polypropylene is a cost-effective medical-grade plastic material and is used where steam-sterilized medical devices are necessary. In addition to resistance to steam sterilization, mechanical performance properties of polypropylene include durability for the number of cycles it can be reused. Its recyclability also makes it an attractive medical-grade plastic.
- Polyethylene is a versatile, durable thermoplastic with a wide range of applications. Its high impact resistance and resistance to chemicals, along with low moisture absorption make it a choice medical grade plastic. It doesn’t fade nor retain dangerous bacteria and can withstand harsh cleaning agents. It is often one of the materials used in medical implants because it is a porous synthetic polymer that is biologically inert and does not degrade in the body.
Medical Plastic Products That Meet Market Demands
Medical plastic products are revolutionizing the healthcare industry. The growth of polymers in medical devices has transformed the marketplace, with plastic medical devices steadily replacing other materials such as glass, ceramics, and metals, wherever applicable. Medical grade polymers can vary, but have certain characteristics and properties in common, along with meeting regulatory standards and requirements.
When it comes to deciding between reusable medical devices and single-use medical devices or disposable medical supplies, healthcare professionals have to consider the pros and cons of each. For starters, medical devices also cover a wide range of categories. Whether the medical devices is for single-use or a reusable item, it can be any equipment or piece of equipment, or an instrument, with medical applications, designed for and used in assessing, monitoring, diagnosing and treating patients. (more…)

A medical OEM is an acronym for an Original Equipment Manufacturer that produces medical devices. Medical OEMs are an important part of the medical device manufacturing industry. Because medical devices come in a wide range of instruments, apparatuses, implements, objects, implants, machines, even software or types of material, the medical device manufacturing industry is a vital sector of the economy. One that provides services and products utilized by and in support of global healthcare systems. The medical OEM functions similarly as other OEMs, but there are a number of FAQs that define its unique role in the medical device industry. Specifically:
What is a Medical Device OEM?
A medical device OEM manufacturer designs, engineers and manufactures complete products and systems. Some OEMs also manufacture sub-assemblies or component parts. Other manufacturers purchase these and assemble them into their products. It is not unusual for OEMs to perform all of these functions: parts manufacture, sub-assembly, and final production.
Such a wide range of medical devices constitutes the medical device manufacturing industry, one that continues to expand to meet the demand of global populations. Medical OEMs produce the instrumentation and equipment specifically designed for diagnoses and treatments of patients worldwide. Whether it’s surgical instrument trays or implants, simple tongue depressors, or advanced imaging systems, medical device OEMs plays an important, if not vital, role in the healthcare industry.
What is the Meaning of OEM?
An Original Equipment Manufacturer (OEM), broadly defined, is a company that produces components for itself, or for use by another company (sometimes referred to as a VAR, i.e., value-added reseller) to sell or incorporate into another product for resale. A component can be a part or finished product depending on the needs and specifications of the reseller. OEMs provide Contract Manufacturing (CM) services for developers, designers, entrepreneurs, individuals, and companies who do not have the capacity or production means to manufacture a device or component. This is a particularly common method of production in the medical device industry. OEM CMs can manufacture, assemble, package and ship on behalf of their clients.
What is a Medical OEM?
A medical OEM is a company that manufacturers medical related equipment or devices, finished or component parts. Because of stringent regulatory oversight of the medical device industry, medical OEMs offer a broader range of product services such as validation and verification, design and engineering, etc., in conjunction with their manufacturing capacity.
What is Medical Device Outsourcing?
Outsourcing is the practice of one company contracting out operational activities and services to another company. It is a method which allows one company to cut operational and manufacturing costs by contracting those functions to another company to perform for less. As a business model and market, global medical device manufacturing outsourcing is a standard practice in the industry. Outsourcing can include any number of services that range from product testing to design and development to production, packaging, and distribution.
What is Own-Brand Labeling?
An OBL, or Own-Brand Labeler, is a practice where products are manufactured by an OEM and then sold under the name, trademark, or brand label of another company. OBL is also referred to as a Private Labeler, and in the UK by statute as “Virtual Manufacturing.” In the medical device industry, OBLs are not part of or involved with the manufacturing of a particular device from an OEM. The responsibility of the design, validation, and verification, production, manufacturing, and packaging are provided by the OEM. The OBL then purchases the components or finished products from OEMs to market and sell under their own name.
If you want to learn more about medical device OEMs, read a former blog post What is a Medical Device Contract Manufacturing Service Company?
As with any industry, medical device manufacturers are governed by industry standards and government regulations. Chief among these standards is ISO 9001—the international standard for quality management systems for companies, businesses, and organizations. ISO first published the standard in 1987. Updated versions were published in 1994, 2000, 2008, with the newest version being ISO 9001:2015. The ISO 9001 quality management standard applies to a wide range of industries, suppliers, and services. A short list includes pharmaceuticals, oil and gas, mining, energy, electronics, steel, banking, health care, hospitality, utilities, biotechnology, and the manufacturing of medical devices. With regard to the latter, many countries rely on ISO standards in regulating medical devices to develop similar quality system requirements internationally. Yet, does the ISO set inspection standards for medical devices with regulatory agencies globally? In the U.S. what role does the FDA play in medical device regulation and compliance? And what exactly is the ISO? And how does ISO 9001 affect the medical device industry? It can be confusing. Read on to answer these and other FAQ’s relating to ISO 9001:

What is the FDA?
The Food and Drug Administration (FDA) is a U.S. government agency responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological goods and products, blood products, and medical devices, as well as to ensure the safety of the food supply for people and animals, dietary supplements, cosmetics, and products that emit radiation. It is the regulatory body for the medical device industry.
What is the ISO?
ISO is the International Organization for Standardization. It is an independent, non-governmental international organization with a membership of 162 national standards bodies. It establishes guidelines, requirements and specifications for products, services and systems, to ensure quality, safety and efficiency. It also works to harmonize its standards with those of the regulatory agencies and bodies around the world.
What is ISO 9001?
ISO 9001 is the International Standard that promotes and defines the criteria for the development, implementation, and effectiveness of a quality management system for companies, businesses, and organizations. It is the only standard in the ISO 9000 series of standards of quality management guidelines and tools that is certifiable for companies and organizations. To date, over one million companies and organizations in over 170 countries have been certified to ISO 9001. The standard focuses on seven customer-oriented quality management principles to ensure consistent, quality services and products for customers, that the implementation of will also, in turn, reward businesses with success. The ISO 9001 principles are customer focus, leadership, engagement of people, process approach, improvement, evidence-based decision making, and relationship management. It is important to note that ISO 9001 is not a product standard, but a tool to control your processes and a quality system that your end product should meet. Also, ISO 9001 is not a membership group. A company or organization doesn’t ‘join’ ISO 9001. It must meet the criteria as defined in the certification requirements and pass the ISO audit. Once passed, the ISO registrar issues an ISO 9001 Certificate good for a three year period.
Why was ISO 9001 Developed?
Developing and establishing international standards provides a benchmark for quality management systems to meet. A consequence of bureaucratic regulation is a tendency toward a piecemeal approach to address evolving system requirements. Quite often, an overarching consistency is lacking, along with other inefficiencies, be it the private or public sector. ISO 9001 was developed to avoid such inconsistencies and to achieve a uniformity of objectives for quality management systems.
What is the influence of ISO 9001 on Medical Devices?
The entire scope of ISO 9001 is used by all companies that design, produce, manufacture, process, pack, label, and ship medical devices are required to comply with governmental regulations based either directly or indirectly on the ISO 9000 series. Most medical device manufacturers are ISO 9001 certified, along with other certifications.
Does the Medical Devices Industry Have an ISO?
The medical device sector follows the guidelines and requirements of ISO 13485. It is the international standard for quality management systems that established requirements for the medical device industry. ISO 13485 is designed and intended to function with other management systems around the world, and by doing so, work more efficiently and transparently with each other. The standard functions as a set of supplementary requirements used in conjunction with ISO 9001, which encompasses requirements specific to medical device manufacturers. Recently, the FDA announced its intention to use ISO 13485 as the basis for its quality system legislation.
It is well-known that urine specimen collection is a common method that physicians utilize to ensure their patient’s health. For most people, giving a urine sample is a routine part of any physical. More than a measure of one’s health and well-being, it can be vital in diagnosing the presence or observing the condition of disease. For example, a urine test can show whether you may have diabetes or are suffering from a urinary tract infection. Urine specimen collection is also used to detect or monitor illegal drug use in many jobs, professions, and athletic competitions. Only blood samples are used more often than urine samples for analysis in clinical laboratories. (more…)