We offer white room and cleanroom packaging for sterile devices, diagnostics, and lab consumables. Whether it’s a private-label product or incoming Work in Progress (WIP), we’ll work with you to ensure your medical device packaging is compliant, functional, and ready for market.
Have a design? Need one?
Whether you have a packaging spec or need help developing one, we will ensure it fits your product, process, and compliance goals.
Custom and Private Flexibility
We support a wide range of plastic packaging solutions and private label formats, tailored to your product, regulatory requirements, and brand.
Packaging for Performance
The right packaging protects your product, supports sterilization, and enhances usability. We’ll help you choose what works best.


We deliver plastic packaging solutions that comply with ISO 13485:2016 and FDA 21 CFR Part 820, supporting labeling, traceability, and cleanliness standards to help ensure product approval and successful market entry.

You won’t find a one-size-fits-all approach to plastic packaging solutions here. We partner early to understand your packaging specs, storage needs, and sterilization workflows and adapt accordingly. If you need a capability we don’t have in-house, we’ll source it or build it to meet your needs. For shrink-wrap, clamshells, multi-packs, and retail-ready display assembly, we research each request to identify the most viable solution.
We start by understanding your product, its packaging and labeling needs, and any downstream processes to recommend the best-fit custom packaging solution.
Based on your regulatory needs, we package your product in a white room, Class 8 cleanroom, or modular space, ensuring compliant sterile packaging for medical devices.
We source packaging materials, assemble your product, and apply custom or barcoded labels, ensuring lot traceability across the entire medical device packaging workflow.
Each unit undergoes quality review and documentation before leaving our facility, ensuring traceable, compliant plastic packaging solutions every single time.
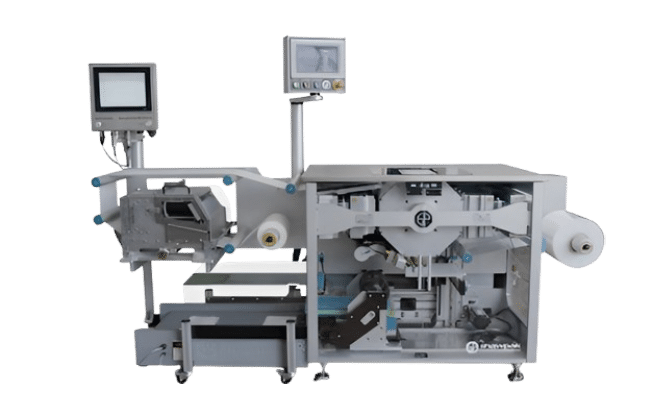
Our new form-fill-seal packaging line gives you more flexibility and faster turnaround. Instead of relying on large inventories of pre-printed bags, we use Tyvek® and film roll stock to form, fill, seal, and print packaging in-line, reducing lead times, eliminating overstock, and simplifying changeovers across SKUs.
We offer injection molding and injection blow molding, which are optimized for precision, compliance, and scale in cleanroom environments.
We operate an 8,000 sq. ft. ISO Class 8 cleanroom with injection molding and injection blow molding, built for high-quality, compliant component production.
From manual to automated assembly in our white room and cleanroom, we deliver finished components with precision, speed, and traceability.

We’re more than a supplier—we’re a strategic partner. As a family-owned company, we move fast, stay flexible, and invest in what matters.
Our collaborative mindset and technical depth help OEMs turn great ideas into manufacturable solutions.
Learn more about usWe help OEMs bring complex molded components to life—with the care, speed, and flexibility they depend on.
We provide custom packaging solutions for medical devices and diagnostics, including Tyvek® peel pouches, tear pouches, and thermoform trays. We also offer white room and Class VI modular cleanroom assembly, private labeling, and support for both bulk and individual packaging formats.
Yes. We offer sterile packaging for medical devices, including lot sterilization support and contamination prevention. Our packaging environments (Class 8 cleanroom and white room) are controlled to meet the needs of RNase/DNase-free products and ensure compliance with regulatory and microbiological standards.
Absolutely. We support customer-led and co-developed custom packaging solutions—including private label, serialized packaging, and incoming work in progress (WIP). We also offer in-line printing via our new form-fill-seal packaging line for flexible, scalable production, helping reduce inventory and lead times. We also support custom label artwork, serialized barcoding, and product-specific use instructions as part of our cleanroom packaging workflow.
From reagent containers to lateral flow housings, we manufacture IVD components in cleanroom environments to support diagnostic precision.