IVD contract manufacturing has been central to our services for over 40 years. Our custom-molded medical device components support the full diagnostic workflow, from sample preparation to signal detection, with quality, precision, and a commitment to advancing your diagnostic goals.
Built for Better Results
In Our Element
A Trusted Partner
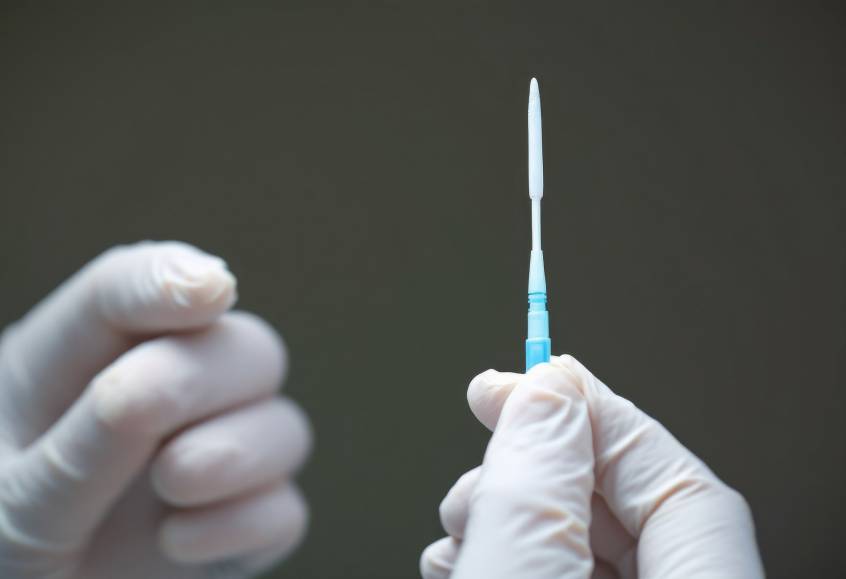

We manufacture IVD components with tight tolerances and scalable quality. From IV set components to custom cartridges and housings, we help ensure reliable performance from prototype to production.

We tailor our IVD manufacturing process to your assay’s performance and regulatory needs. From selecting bio-compatible materials to holding micron-level tolerances, we ensure your diagnostic components integrate seamlessly, delivering confidence, consistency, and compliance.
We collaborate early to align on assay needs and manufacturability. Our IVD manufacturing expertise guides material selection, part design, and production feasibility.
Our engineers create precision tooling and optimize processes to meet your medical device component’s specs and quality standards in IVD contract manufacturing.
We mold IVD components, including IV set components and housings, in our ISO Class 8 cleanroom, built for consistency, compliance, and scalable production.
From cleanroom assembly to packaging and inspection, we manage each step of IVD manufacturing to deliver reliable diagnostic components on time, every time.
We support everything from prototyping to high-volume production of IVD components in our ISO Class 8 cleanroom. Whether you’re scaling a new assay or expanding capacity, we deliver consistent, compliant results. From IV set components to lateral flow housings, PCR vessels, and sample transport containers, we help you grow with confidence through every phase of IVD contract manufacturing.
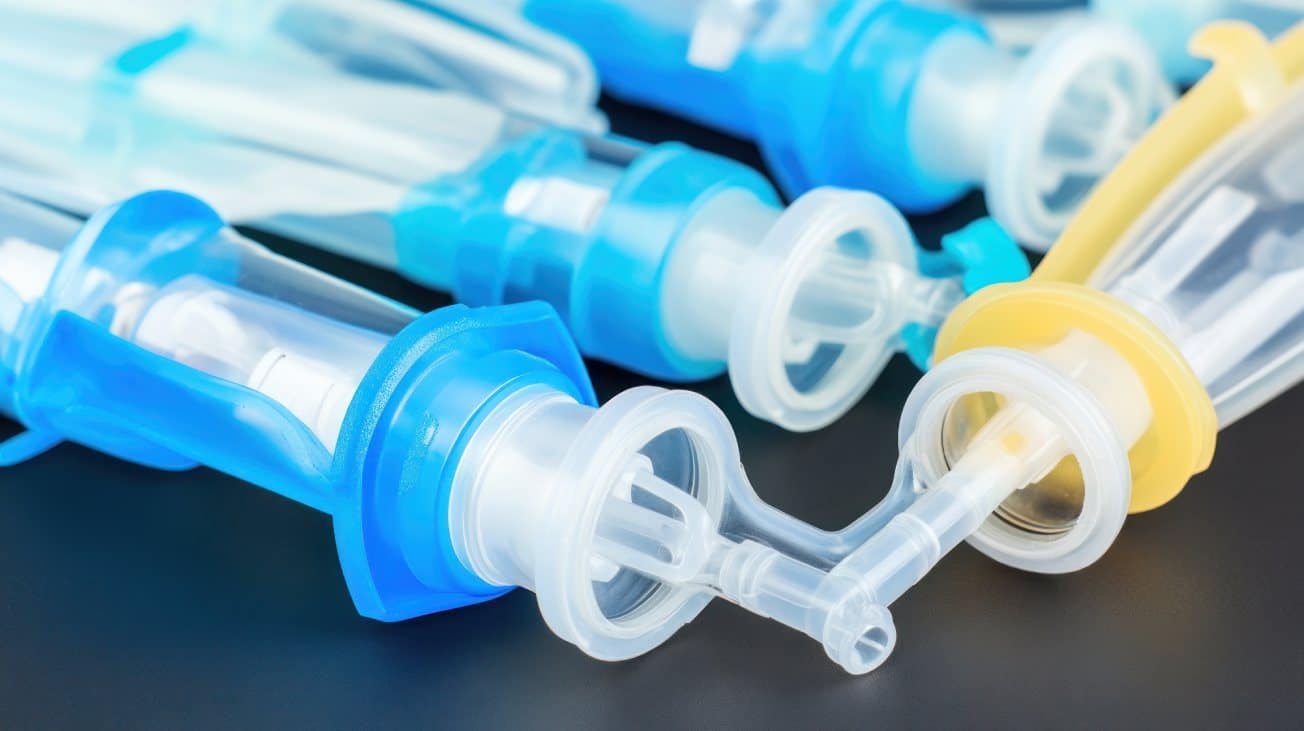

We specialize in custom-molded medical device components used throughout the diagnostic workflow, including housings, fluid channels, sample prep parts, and IV set components. Whether you're developing a point-of-care cartridge or a complex lab-based assay, our IVD manufacturing capabilities are built to meet tight tolerances, bio-compatibility standards, and regulatory requirements. We work closely with your team to ensure each part supports the performance and reliability that your test demands.
Yes. Our IVD contract manufacturing is ISO 13485 certified, and we operate an ISO Class 8 cleanroom for the production of diagnostic and medical device components. These certifications reflect our commitment to quality, consistency, and compliance at every stage. We understand the critical role IVD components play in healthcare and take that responsibility seriously. From documentation to delivery, we’re a partner you can rely on for regulated, high-performance manufacturing.
We recommend involving us as early as possible, ideally during the design phase. That’s when our team can provide the most value, helping you make smart decisions about materials, tolerances, tooling, and scalability. Our collaborative, solutions-focused approach is designed to reduce risk, streamline development, and set your project up for long-term success. As a flexible, privately-owned IVD manufacturing partner, we move quickly and adapt to your needs without compromising on quality or service.